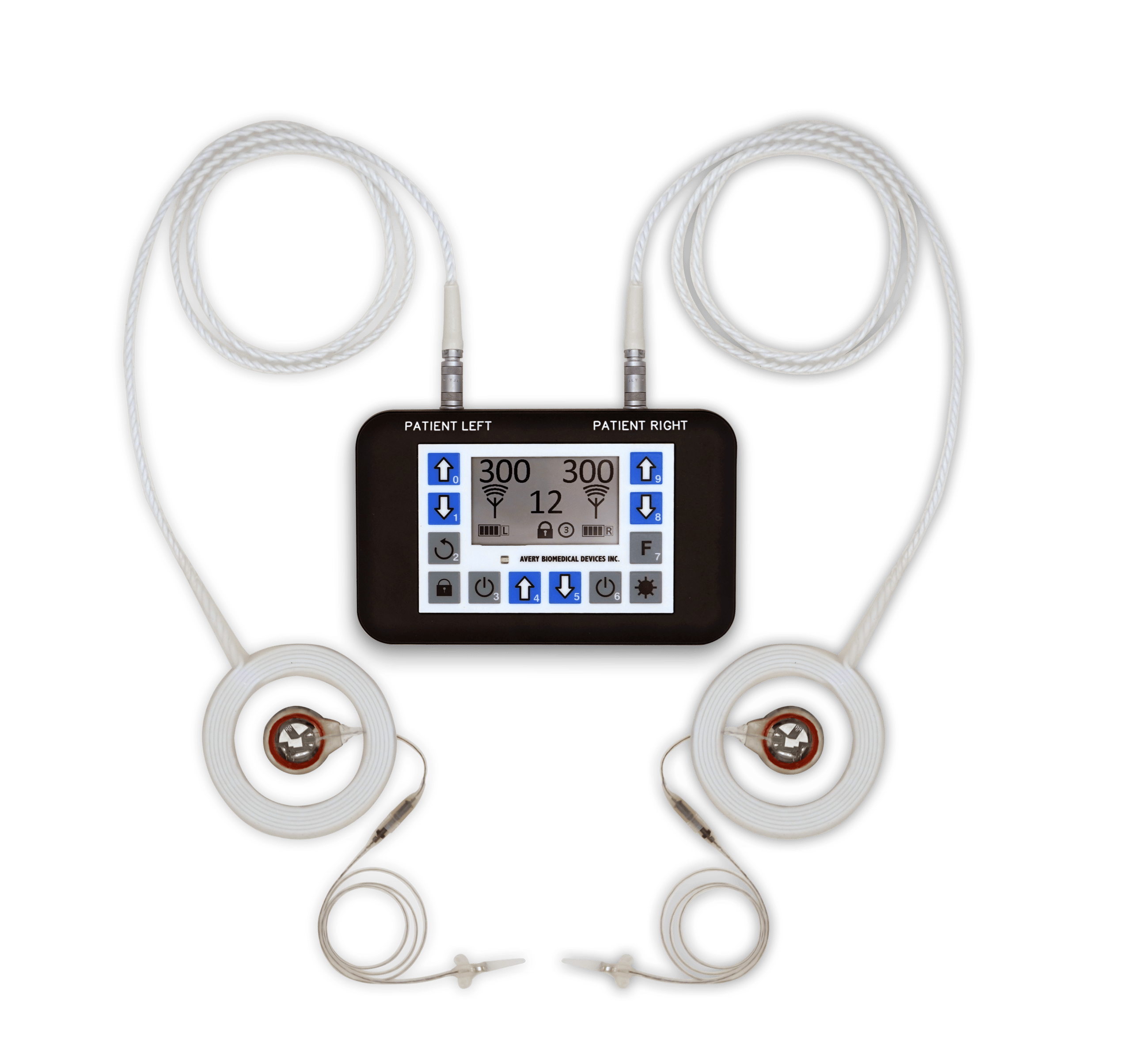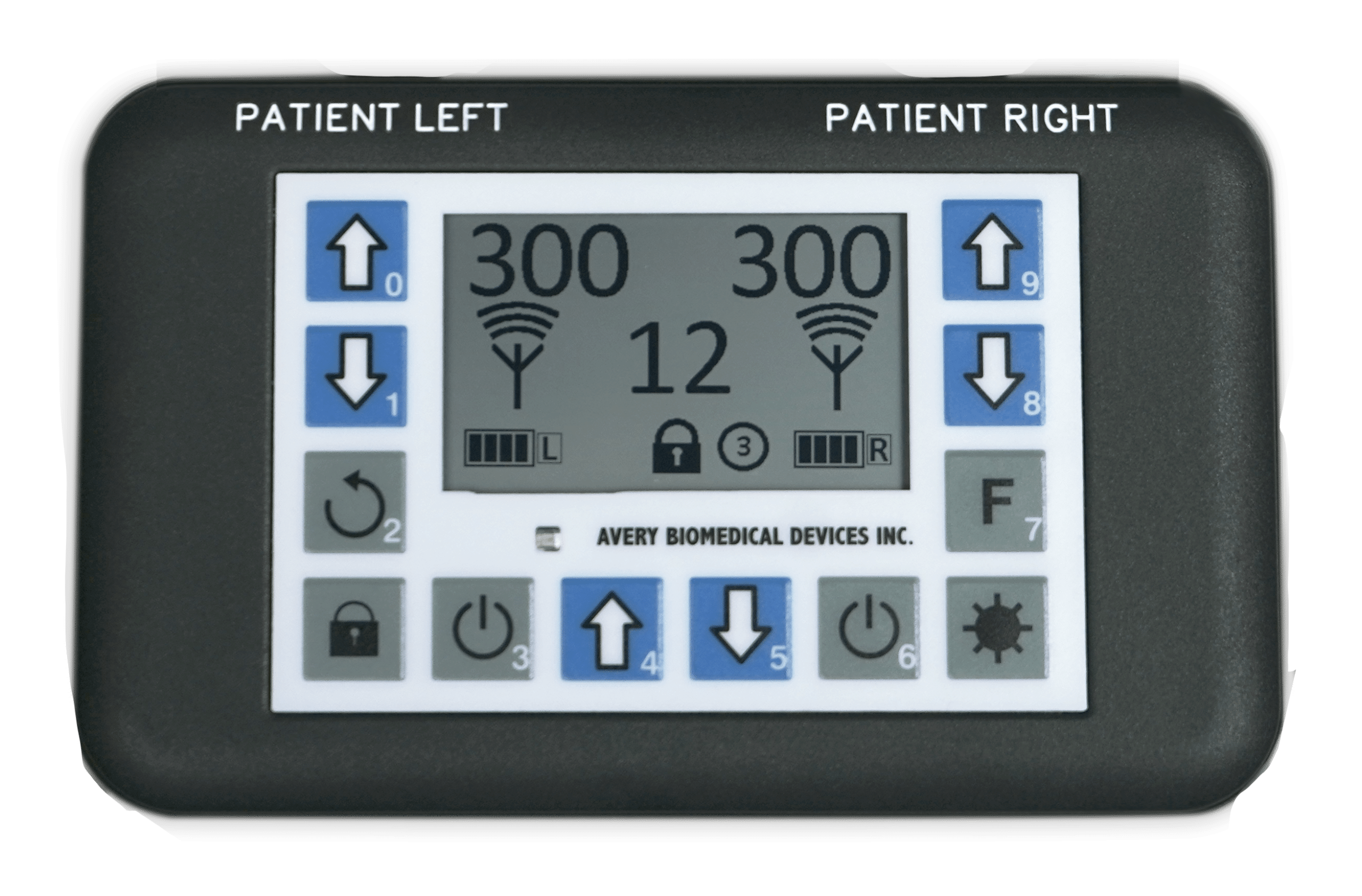
The Spirit Diaphragm Pacing Transmitter is CE Marked under the European Active Implantable Medical Device Directive as of 2017, and received full FDA Approval in November 2019. The transmitter is lightweight and features a backlight for nighttime viewing, clear graphic display, touch controls, and digital precision. For safety, it is also water resistant, has keypad locks, utilizes audible and visual alarms, and features bilateral redundancy.
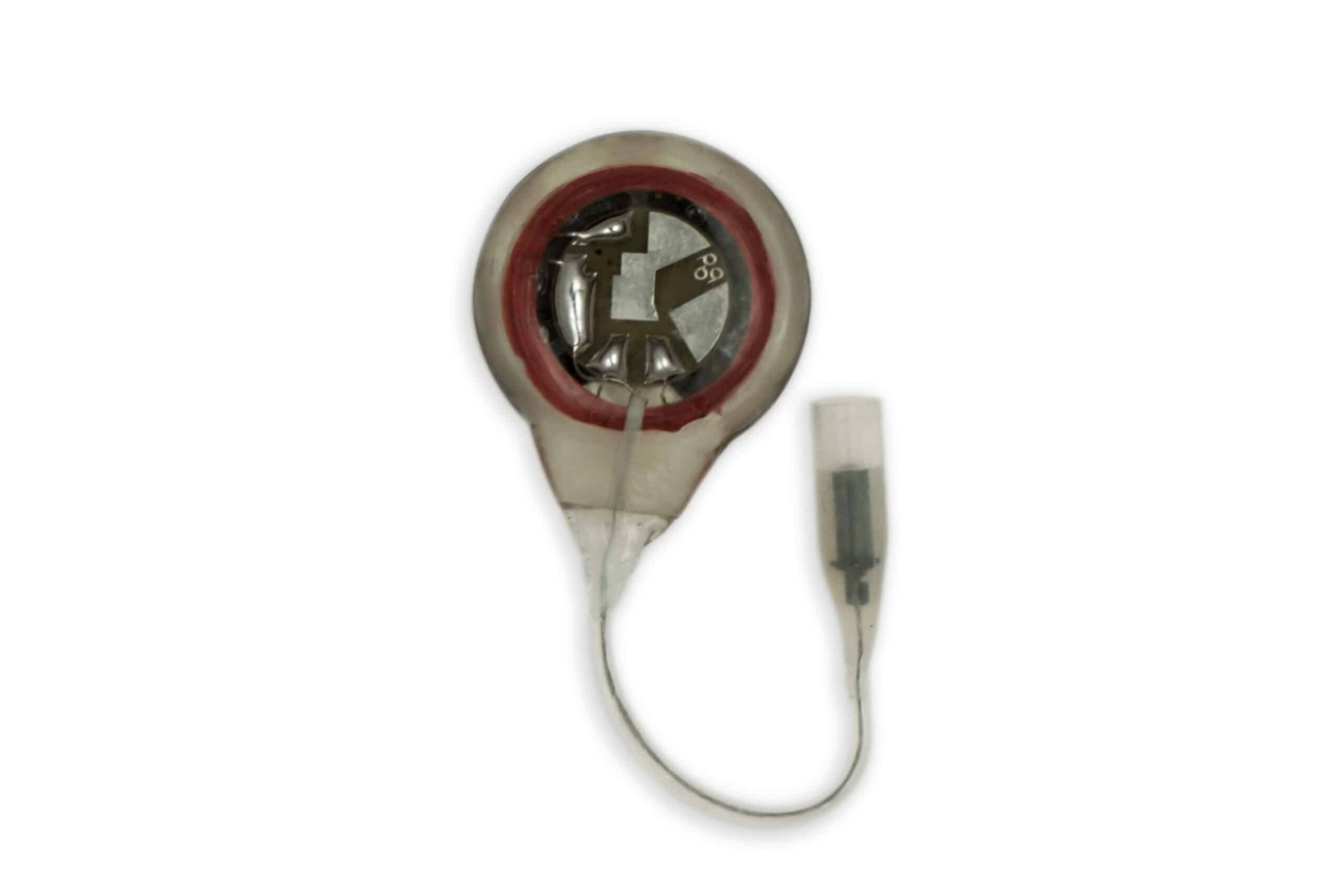
The implanted I-110A receiver is a small disc-shaped device that contains electronic circuitry embedded in epoxy resin and coated with silicone rubber. Each receiver converts the stimulus energy from the antennas into distinct stimulus pulses and transfers it to the electrodes. The receiver I-110A has a single connector, uses an integrated anode (horseshoe-shaped) plate and the circuit.

The implanted electrode is comprised of highly flexible, stainless steel strands insulated by silicone rubber, with a platinum nerve contact on one end and a connector that mates to the receiver on the other end. Each electrode accepts the stimulus pulses from the receiver and transfers it to the phrenic nerve, causing the diaphragm muscle to contract. The electrode (Model E-377-05) is composed of a single wire assembly.

In March 1998, the U.S. Food and Drug Administration gave premarket approval (PMA) to the new Mark IV external transmitter for adult and pediatric patients who have lost neurological control of respiration. FDA premarket approval is required before Class III medical devices can be commercially distributed in the United States.
In advance of FDA approval, the Mark IV had been distributed in 24 countries worldwide. The Mark IV qualified for the CE Mark under the European Active Implantable Medical Device Directive in 1995.

An external antenna is worn over each implanted receiver and sends power and radio signals from the transmitter to the receiver transcutaneously. This radiofrequency coupled design means no wires or plugs protruding from the skin, and no batteries in the implant requiring periodic replacement.
An antenna is a durable disposable item requiring periodic replacement. It is recommended that antennas be replaced prophylactically every six months. Antennas are available in one-meter and two-meter lengths.
Antennas do not contain latex. Additionally, no latex products are used in its manufacturing. Instructions for the use and care of antennas can be found below.