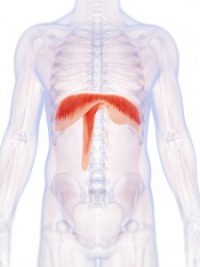
The Diaphragm
The diaphragm is a large flat muscle positioned within and across the bottom of the rib cage. Shaped like an open parachute, it separates the lungs from the stomach area. The diaphragm performs an important function in respiration. When it contracts, air is drawn into the lungs. When the muscle relaxes, air is expelled.
DIAPHRAGM PARALYSIS
Diaphragm paralysis is the loss of control of one or both sides of the diaphragm. This causes a reduction in lung capacity. Patients with diaphragm paralysis may experience shortness of breath, headaches, blue lips and fingers, fatigue, insomnia, and overall breathing difficulty.
THE CAUSES
Causes of diaphragm paralysis include, but are not limited to:
- Spinal cord injury
- Direct trauma to the phrenic nerve
- Central neurological disorders, which may result from brain or brainstem stroke, disease such as meningitis, or from insect bites
THE TREATMENT
Patients with a paralyzed diaphragm who have functioning phrenic nerves can seek treatment with the Avery Diaphragm Pacing System System.
The diaphragm pacing system uses surgically-implanted electrical impulses to rhythmically stimulate the phrenic nerve which helps restore breathing function. The surgery is performed by placing an electrode under the phrenic nerve, either in the neck or in the chest.
THE BENEFITS
Patients with diaphragm paralysis who use the Avery Diaphragm Pacing System System may experience improved respiratory function and lower infection rates. Other benefits of the diaphragm pacing system include improved mobility, normalized breathing and speech patterns, long-term decreased treatment costs, ease of eating and drinking, and improved quality of life.
Contact Avery Biomedical Devices to learn more about diaphragm paralysis and the benefits of the Avery Diaphragm Pacing System System.
THE AVERY DIAPHRAGM PACING SYSTEM SYSTEM
The Avery Diaphragm Pacing System System is the only diaphragm-pacing system with full pre-market approval from the USFDA and CE marking privileges under the European Active Implantable Medical Device Directive for both adult and pediatric use. In addition, its system of using small implanted radiofrequency receivers rather than electrode wires that pass directly through the skin may decrease a patient’s risk of infection and ongoing wound care management issues.
Content reviewed July, 2024 by Dr. Don Headley, M.D.
Dr. Headley is an otolaryngologist (ENT) with almost 40 years of experience in medicine. He is a graduate of George Washington University School of Medicine and Health Science, has been affiliated with St. Joseph’s Hospital and Medical Center in Phoenix, Arizona, and previously served as assistant professor of Internal Medicine and Surgery and Creighton University Medical School in Omaha, NE.

